

Home || Research Interests || Publications || Group Members || SOP manuals
Project SEED || MSU Chemistry || MSU
![]()
Organic Synthesis
![]()
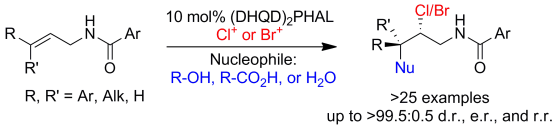
Our research group has recently reported the catalytic asymmetric chlorolactonization of alkenoic acids and unsaturated amides to furnish chiral heterocycles. These reactions are catalyzed by (DHQD)2PHAL in combination with various N-chlorinated hydantoins as the terminal chlorenium sources. Halofunctionalization of different compounds, understanding the mechanism of these transformations and the details of enantioselections are currently under investigation.
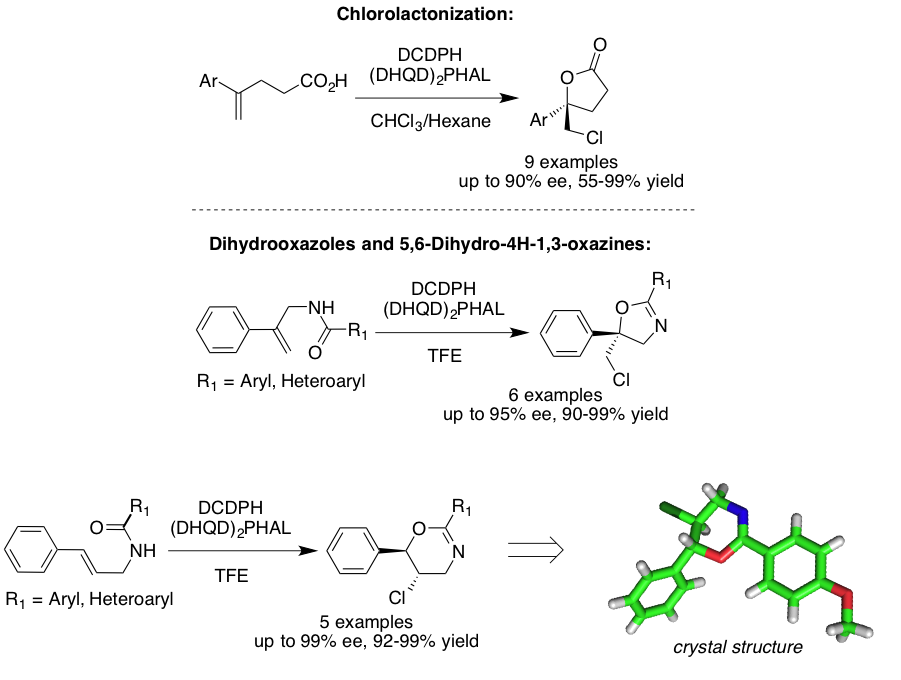
Our research is also focused on dissecting the steps and revealing the mechanistic underpinnings of asymmetric halocyclizations. In collaboration with Professor Ned Jackson, we utilize both experimental results, such as kinetic analyses and thermodynamic meaurements, and theoretical calculations to derive at likely mechanisms provide insight into the origins of regio and enantio selection. These results are then parlayed into the next generation catalyst design.
![]()
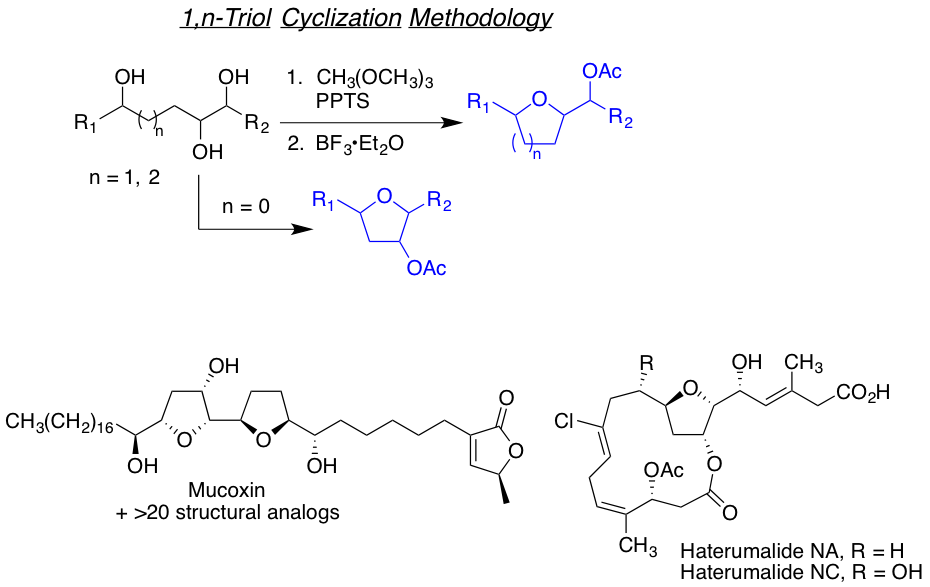
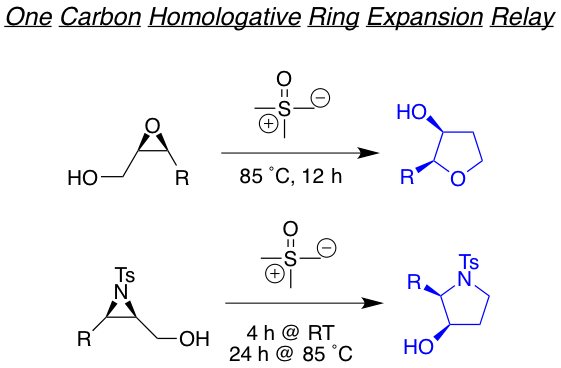
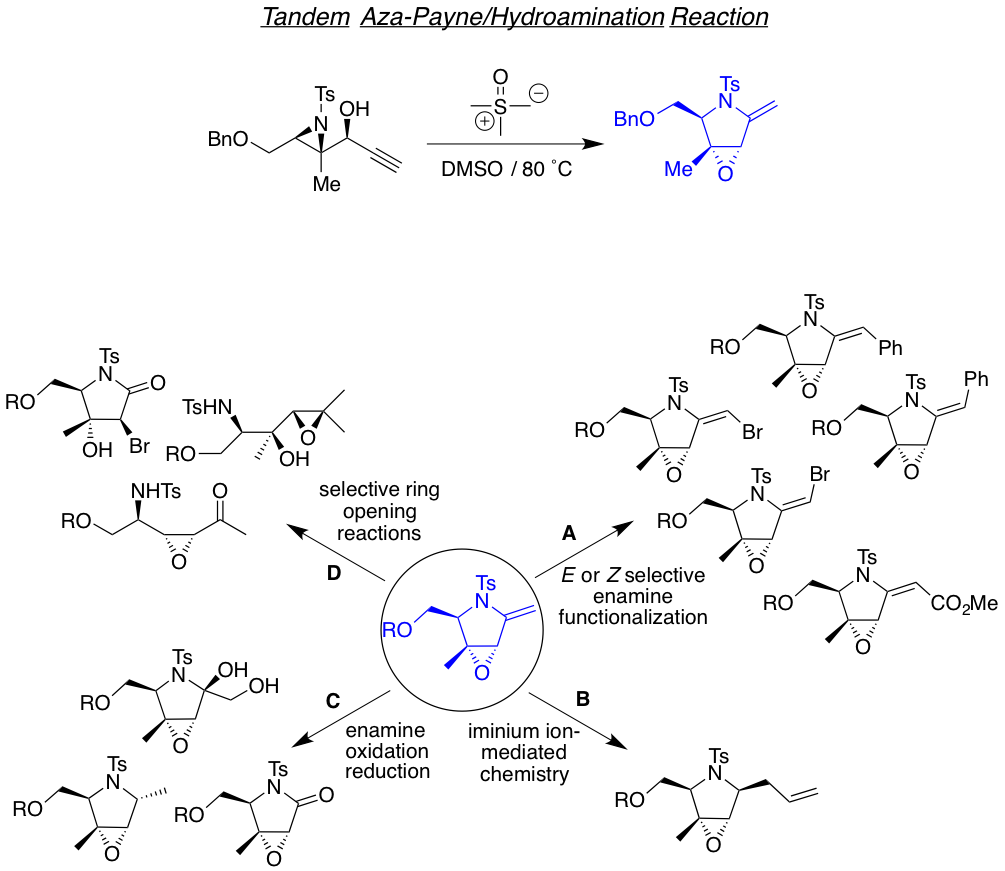
We are also interested in developing reactions that yields highly functionalized heterocyclic motifs in a robust way, with high regio and stereo control, from small and readily available families of molecules. Examples of such are the tandam aza-Payne hydroamination reaction to achieve highly decorated pyrrolidines or 3,4-dihydroxypyrrolidines the one-carbon homologative ring expansion relay chemistry, and the 1,2,n-triol cyclization, towards the preparation of a library of complex dihydropyrans.
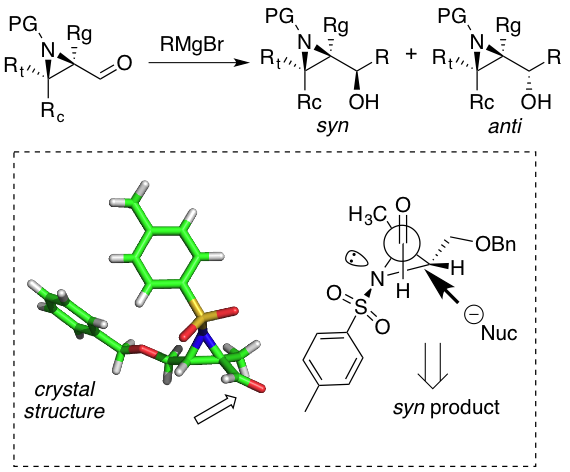
Our venture in the field of asymmetric reactions have motivated us towards a simplistic approach in building small chiral motifs that can potentially trigger a variety of useful transformations with a high level of enantiocontrol.
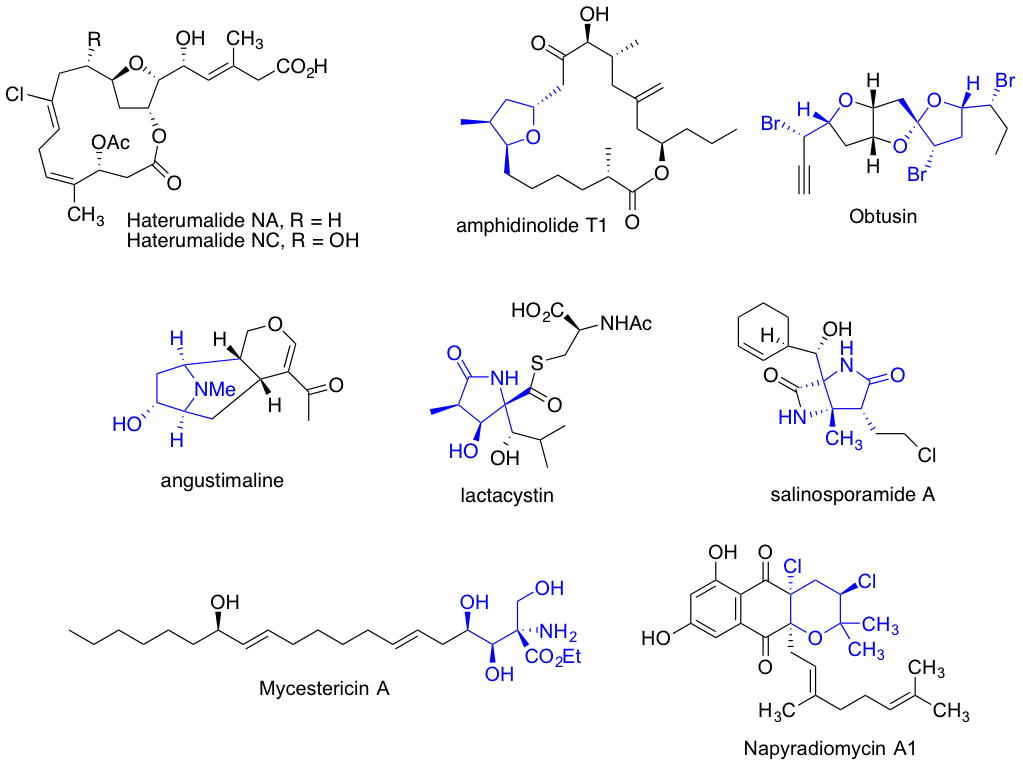
Having all the tools to set up complexity in molecular structures, with high level enantio and stereo control, we are applying these methodologies towards the total synthesis of biologically interesting and synthetically challenging natural product motifs.
Recent Publications
![]()
Catalyst Design
![]()
Total Synthesis
![]()
![]()